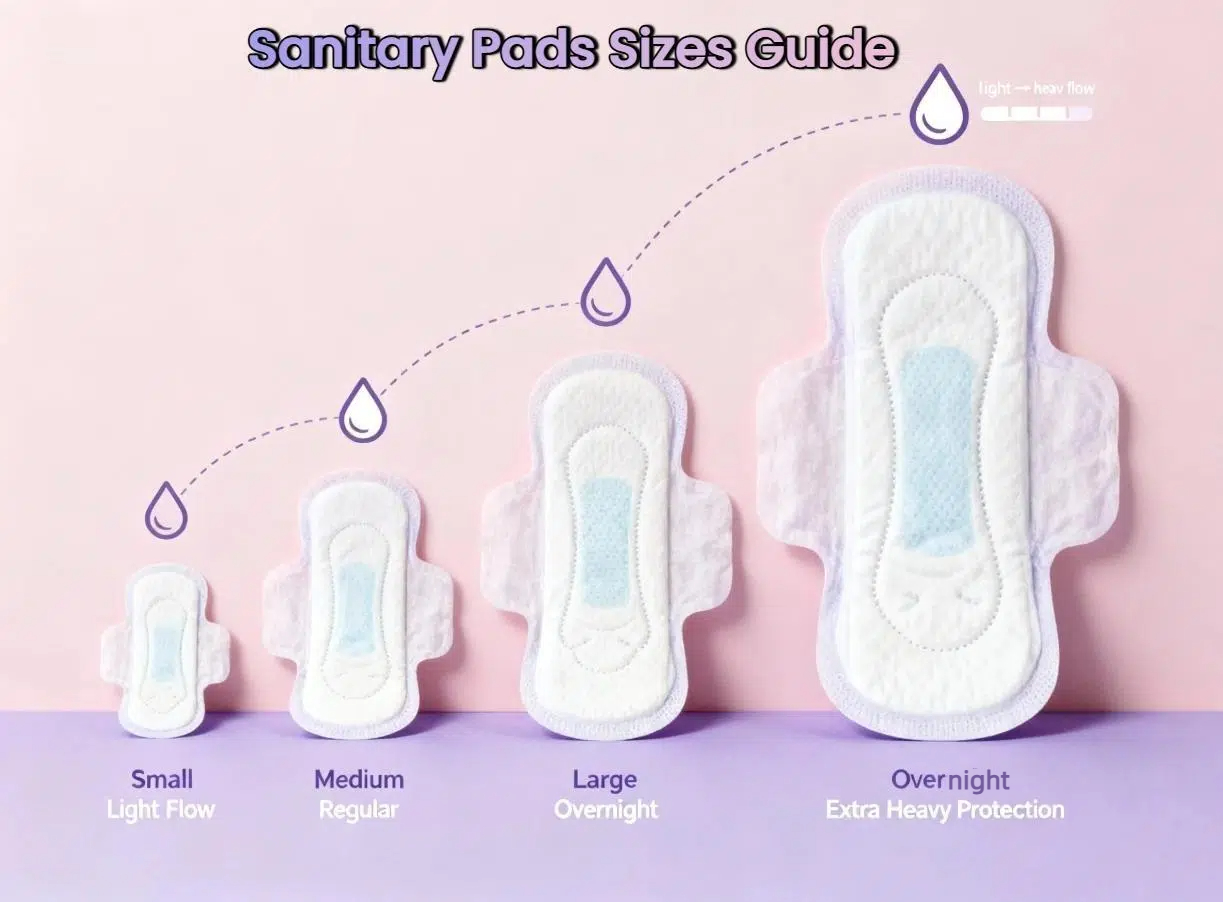
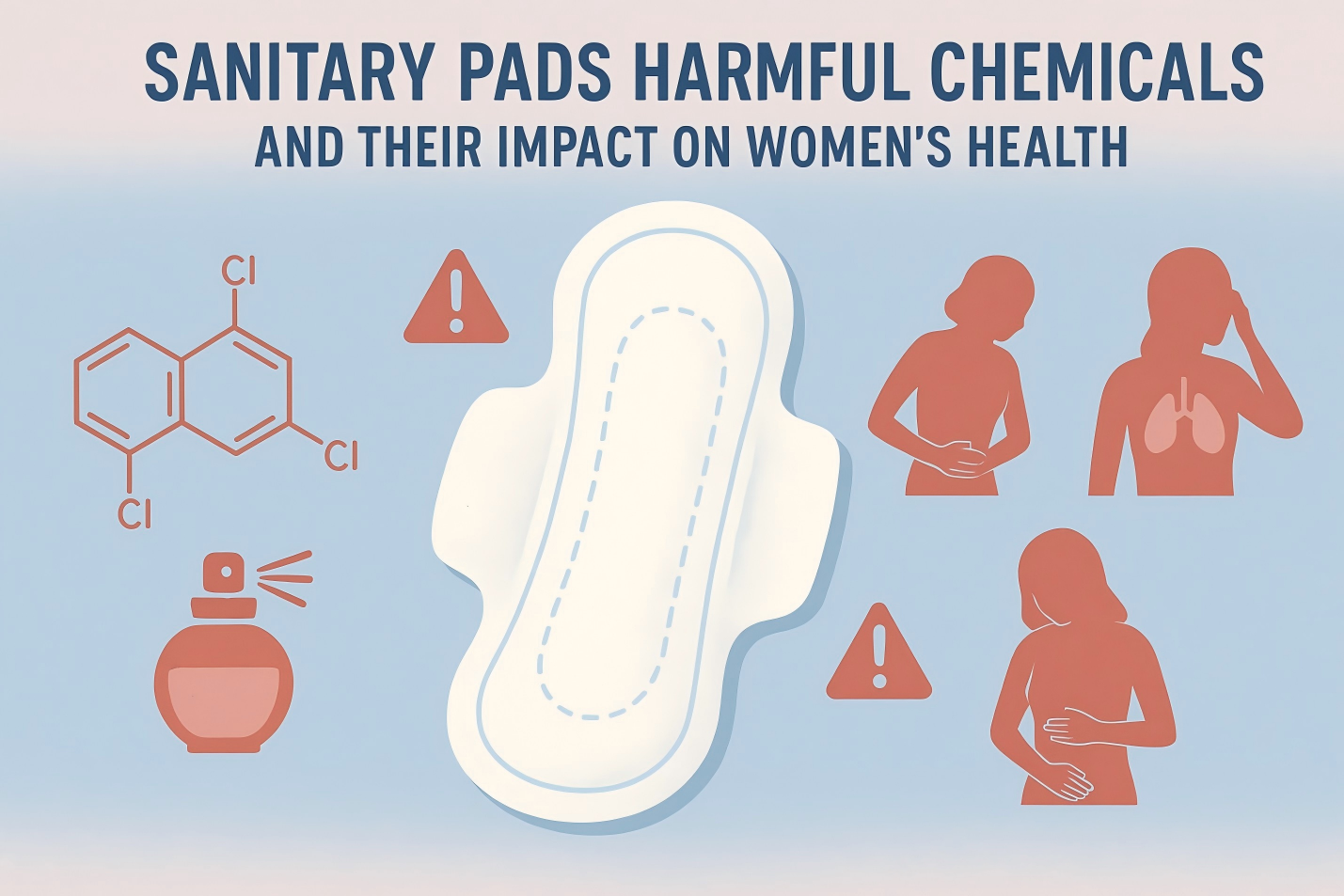
The global feminine hygiene products market is booming. It’s projected to jump from $43.25 billion in 2024 to $73.07 billion by 2032.[1] As manufacturers eye international markets, exporting sanitary pads isn’t just about competitive pricing anymore. Success demands understanding international standards – from optimal sanitary pad design and material safety to absorbency testing, labeling, and packaging regulations. Getting these right builds consumer trust and ensures smooth market access across borders.

Why International Standards Matter?
1. Building Consumer Trust
Trust is everything in feminine hygiene. When you comply with recognized standards, you’re telling consumers: “We’ve got your back”. Standards prove your products have been rigorously tested and actually help your brand position itself as trustworthy in highly competitive markets.
2. Unlocking Market Access
Think of regulatory compliance as your master key to international markets. Many countries won’t even let your products through customs without proper certifications. Skip the standards, and you might find your shipments heading straight back home.
3. Protecting Public Health
Sanitary pads are intimate health products, so material safety isn’t negotiable. International standards guarantee nasty chemicals remain out of your products. Following these standards not only protects women’s health, but also shows a company cares about production, and making a difference.
Key Standards and Bodies to Know
Navigating the regulatory maze gets easier when you know the key players in sanitary pad design standards:
1. ISO
The ISO develops globally recognized benchmarks for product quality and safety. For feminine hygiene, relevant standards focus on:
- Absorbency and performance testing to verify product claims.
- Material safety evaluations, ensuring pads are free from harmful chemicals.
- Quality management systems (ISO 13485, ISO 9001) that govern how manufacturers maintain consistent product standards.
2. Regional Requirements
Different regions have their own rulebooks that complement international standards:
FDA (USA, Canada)
In the United States, sanitary pads are regulated as medical devices (Class II). To meet FDA requirements, manufacturers must:
- Submit a 510(k) premarket notification proving substantial equivalence to legally marketed products.
- Conduct biocompatibility testing to ensure materials are safe for skin contact.
- Follow Good Manufacturing Practices (GMP) under the FDA’s Quality System Regulation (QSR).
CE (European Union)
In the EU, sanitary pads must carry the CE mark to be sold legally. This involves:
- Demonstrating compliance with the EU Medical Device Regulation (MDR 2017/745).
- Conducting safety and performance evaluations, including chemical and microbiological testing.
- Maintaining a technical documentation file and undergoing audits by a Notified Body.
Core Design and Material Requirements: A Deep Dive
Below are the core design and material considerations that determine whether a product is market-ready.
1. Absorbency and Fluid Management
The most critical performance indicator of a sanitary pad is its ability to manage menstrual flow effectively. Key metrics include:
- Total Absorbency Capacity: How much liquid the pad can hold before leaking.
- Rate of Acquisition: The speed at which fluid is drawn into the pad, preventing pooling on the surface.
- Rewet (Wet-back): How much fluid returns to the surface when pressure is applied, directly impacting comfort and dryness.
The Syngina/Rothwell test is a popular industry standard, using synthetic blood through equipment that mimics actual anatomy for evaluating absorbency performance. They aim to provide objective data on pad effectiveness.
2. Material Safety and Composition
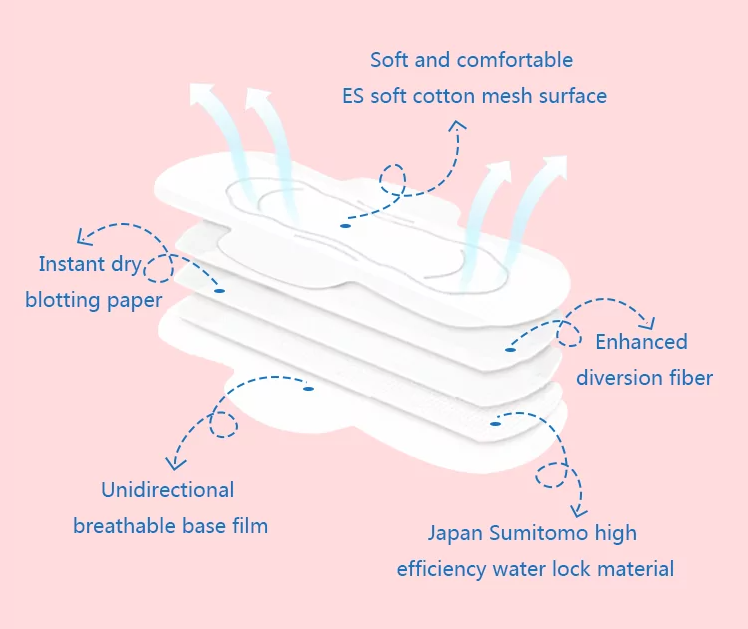
Each layer of the sanitary pad has a distinct role, and international standards require that all components be safe, non-toxic, and skin-friendly.
- Top Sheet: Needs to be soft yet efficient – letting fluid pass through quickly while staying gentle on skin. Common materials like non-woven fabrics, often polypropylene or polyethylene blends, are engineered for comfort and fluid permeability.
- Absorbent Core: This is your pad’s engine, essentially. Super Absorbent Polymers (SAP) plus fluff pulp create the magic combination.
- Backsheet: The outer layer, designed to be impermeable to leaks while ideally allowing some breathability for skin comfort.
- Adhesives: Medical-grade only. They need a strong hold without skin contact and clean removal without residue.
3. Banned and Restricted Substances
Global regulations strictly prohibit or restrict the use of harmful chemicals in feminine hygiene products. Manufacturers must ensure:
- No Harmful Chemicals: Avoid phthalates, dioxins, heavy metals, and formaldehyde entirely. Build in routine checks during production to confirm safety.
- Hypoallergenic Fragrances and Dyes: Fragrances and dyes should be hypoallergenic and safe for intimate use.
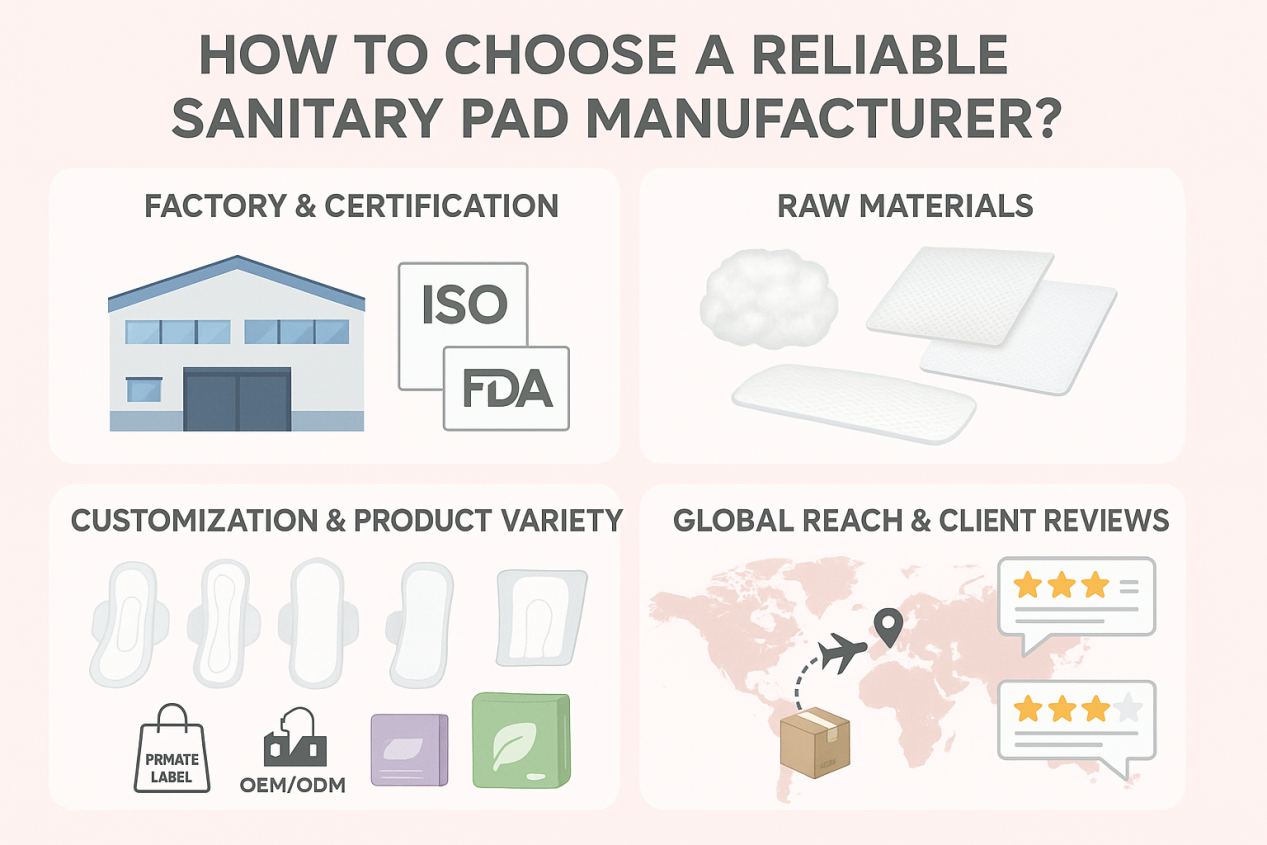
Packaging and Labeling: Your Product’s Global Passport
When exporting sanitary pads, the product itself is only half the equation. Packaging and labeling act as your product’s global passport, ensuring compliance, transparency, and consumer confidence across different markets.
1. Clarity is Key
Regulators and consumers alike demand that packaging information be clear, accurate, and not misleading. Overstated claims, vague descriptions, or missing details can lead to regulatory rejection and loss of trust.
2. Essential Information for the Consumer
Give users what they need to make smart choices. Essential information includes:
- Absorbency Level: Apply the familiar one-to-five droplet icons so users everywhere instantly recognize capacity.
- Material Composition: Listing every sanitary pad material clearly supports sensitive users and builds brand credibility.
- Instructions for Use and Disposal: Simple pictograms explain steps universally, without relying on written language.
- Manufacturing and Expiry Dates: Essential for traceability and quality control. Everyone in the supply chain benefits from proper dating.
- Manufacturer’s Contact Information: Complete contact details show accountability and enable customer support when needed.
3. Language and Localization
Export-ready packaging must be adapted to the target market. This typically means:
- Translating product information into local languages.
- Ensuring cultural sensitivity in imagery and messaging.
- Meeting specific labeling rules (for example, bilingual or multilingual labeling requirements in the EU or Canada).

Conclusion
Exporting sanitary pads successfully means mastering international design standards, regulations, and cultural expectations. Every detail must align with strict international requirements while meeting diverse consumer needs. At Shuya, we understand these challenges. With 40+ years of experience and full compliance with all major standards, we partner with top brands across 60+ countries. Our sanitary pad factory produces 55 million pads monthly, backed by advanced manufacturing capabilities. Visit our website to explore our certified product range and see how our standards expertise can power your global expansion.
References
[1] Available at: https://www.fortunebusinessinsights.com/feminine-hygiene-products-market-103530